

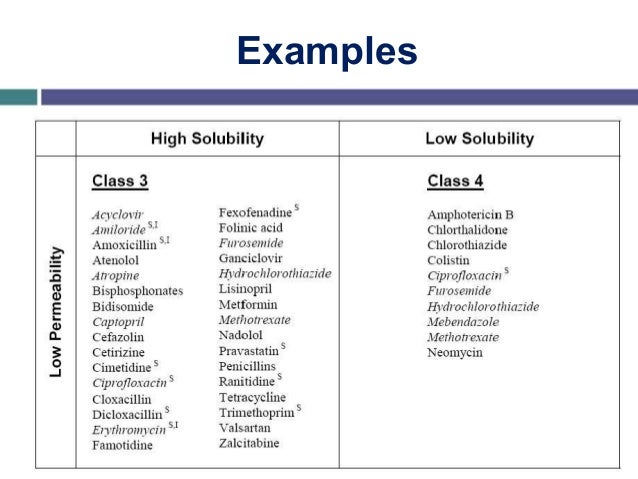
OSP: Please tell us about hot-melt extrusion, how it can improve dissolution, and how HME solves a lot of these challenges.Īmeya Deshpande, formulation scientist, AvomeenĪD: HME is a common technique used for transforming poorly soluble crystalline APIs into amorphous, soluble forms to make them more bioavailable. It can also increase the cost of medication, expose patients to additional risks of adverse effects, and make oral medications taste unpleasant, which can reduce adherence. For example, if the drug is highly potent, it can produce toxic effects in line workers. Pharmaceutical developers often compensate for the low solubility of APIs by increasing the API concentration in their batches however, this practice poses health risks to both production personnel and patients. About 80% of all pharmaceutical products show low solubility in water, which reduces their absorption in the body and poses a significant challenge to pharmaceutical developers looking to produce safe and effective drugs. Could you please share with us the various challenges this poses to pharma developers and drug companies, and how companies typically try and overcome these challenges?ĪD: Active pharmaceutical ingredients (APIs) in solid oral dosage forms such as tablets are only as potent as they are soluble in aqueous solutions since solubility directly impacts the bioavailability of the API. OSP: You point out many APIs in drugs don’t readily dissolve in water. Avomeen offers complete, comprehensive scientific solutions that support the entire product development lifecycle, with a focus on growing strategic partnerships.
#BCS CLASS 1 DRUGS MORE PROFITABLE TRIAL#
Throughout each step of product development, Avomeen’s scientists and experts work within a rigorous quality system to ensure products comply with regulatory requirements and consumer expectations. Avomeen’s facility is FDA-registered and DEA-Licensed (schedules 1-5), and its laboratories are also GLP/GMP-compliant, ISO 17025 accredited, and QP authorized for clinical trial material in the EU. The company’s unique approach is singularly focused on applying its expertise and working closely with clients to help transform their biggest goals into real solutions. OSP: Could you please share the ‘elevator presentation’ description of Avomeen-who you are, what you do, key capabilities, and what sets you apart from the competition?ĪD: Avomeen is an accredited, independent contract research organization (CRO) and contract development and manufacturing organization (CDMO), bringing chemists and scientists together to tackle scientific product-development challenges in the biopharmaceutical industry and across other industries and applications. Outsourcing-Pharma spoke with Ameya Deshpande, formulation scientist at Avomeen, about how hot-melt extrusion could help reduce some of the challenges associated with drug formulation, improving bioavailability, and clearing other hurdles.

Many active pharmaceutical ingredients (APIs) don’t readily dissolve in water, which decreases their potency increasing the concentration of the drug increases the cost and could negatively impact everything from the cost of the drugs to the incidence of side effects, and taste of the pills. One obstacle drug developers face is ensuring the bioavailability of the active ingredients in the treatments they create. Tabletting, coating & ancillary equipment.



 0 kommentar(er)
0 kommentar(er)
